Chimeric antigen receptor (CAR) T cells are a kind of immunotherapy, genetically engineered to target an antigen on the tumor cells. The use of CAR therapy in cancer, by adoptive cell transfer, had been approved by the US Food and Drug Administration (FDA) for use against acute lymphoblastic leukaemia. T cells were removed from the patient and modified to express receptors specific to the patient's particular cancer through recognizing and killing the cancer cells.
For decades, chemotherapy was the first option for the patients with tumor cells, and outcomes were stagnant. Then the anti-CD20 monoclonal antibodies which work differently than chemotherapy were discovered and improved our survival. The immune-chemotherapy had lasted for approximately ten years, until targeted therapies appeared and improved the outcomes of some patients with resistant lymphomas.
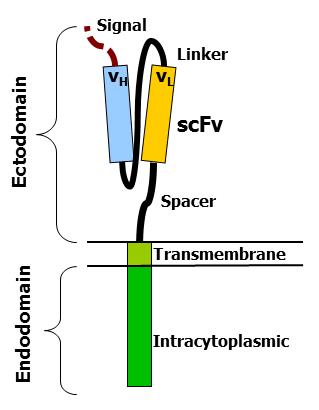
Fig.1 Different components of an artificial TCR (from Wikipedia)
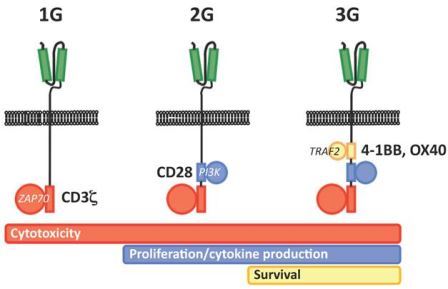
Fig.2 Depiction of three generations chimeric antigen receptors (from Wikipedia)
The earliest CARs targeted a tumor antigen, mostly CD19 in the case of B-cell lymphoproliferative disorders, CD28 or 4-1BB. About CAR T-cell therapy induces responses in between one-half and two-thirds of patients. Approximately one-third of patients had complete response at 3 months after treatment. In the American Society of Hematology meeting on 2016, the reports shown that in the treatment of 51 patients with pretreated heavily pretreated diffuse large B-cell lymphoma (DLBCL) and 11 patients with transformed follicular lymphoma or primary mediastinal B-cell lymphoma, the best overall response rate reached 79%, with a complete response rate of 52%. Especially in the subgroup of patients with DLBCL, these rates were 76% and 47%.

Fig.3 Depiction of adoptive cell transfer therapy with CAR-engineered T cells (from Wikipedia)
However, there still remain several powerful challenges to the broad application of CAR-T cell therapy in the future. Tumor antigen escape was the main challenge for the long-term disease control in B cell malignancies. And the translation to solid tumors would be another significant hurdle. In addition, ineffective therapies in a subset of patients made the therapies inapplicable. For example, in therapies for patients with DLBC or mantle cell lymphoma (MCL), it appeared fall and short. Severe treatment-related toxicities mainly as the on-target/off-tumor recognition are the other obstacle for CAR-T cell therapy beyond hematological malignancies.
Reference
M Pule, H Finney, A Lawson. Artificial T cell receptors. Cytotherapy., 2003, 5 (3): 211–26.
Gallagher, James. "Historic 'living drug' gets go-ahead". BBC. Retrieved 30 August 2017.
P Martin. The use of CAR T cells in diffuse large B-cell lymphoma and mantle cell lymphoma. Adv Hematol. , 2017, 15 (4): 247.
Z Wang, Z Wu, L Yang, et al. New development in CAR-T cell therapy. J Hematol Oncol., 2017, 10 (1): 53.







