The summary was published at this year by Yigong Shi, which provided the currrently important development of the spliceosome.
The spliceosome was a large RNA-protein complex that catalyzed the removal of introns from nuclear pre-mRNA, named as the ribonucleoprotein machinery. And ribonucleoproteins (RNPs) could mediate key cellular functions, like gene expression and regulation. Although most RNP enzymes were stable in composition and harbor preformed active sites, the spliceosome followed fundamentally different strategies. The spliceosome could provide accurate recognition and flexible splicing during alternative splicing by various compositional and structural dynamics in substrate-dependent complex assembly, catalytic activation, and active site remodeling [1-2].
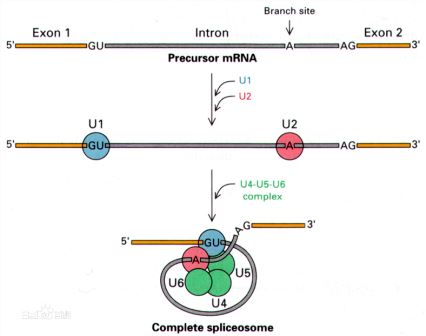
But its atomic structure had not been resolved for nearly 40 years. However, the yeast spliceosome was come into light in the cryogenic electron microscopy structure now. With the atomic or near-atomic resolutions, the yeast spliceosome was found having four distinct conformational states. At the active site, there were two catalytic metal ions being coordinated by the U6 small nuclear RNA (snRNA). They also catalyzed both the branching reaction and the exon ligation. The fully assembled spliceosome had U5 and U6, along with 30 contiguous nucleotides of U2 at its 5′-end. Prp8 and 16 core protein components supported the rigidity of RNA elements, to maintain the conformation in all structurally characterized spliceosomes. The mobile site was only the sequences downstream of nucleotide 30 of U2 snRNA, which delivered the intron branch site into the close proximity of the 5′-splice site for the branching reaction. The addition structural rearrangement depended on the exon ligation, and the lariat junction recruited the 3′-splice site and 3′-exon at the active site. The spliceosome is proven to be a protein-directed metalloribozyme [3].

The researching team of Prof. Shi previously reported these great studies were at 2015 on Science. Their researches were not only improved the precision from 5.9 A to 3.6A but also analyzed the real spliceosome, which was the first time for human to observe at near-atomic resolutions.
Reference
1. AI Lamond. The spliceosome. Bioessays, 1993, 15(9): 595.
2. MC Wahl, CL Will, R Lührmann. The spliceosome: design principles of a dynamic RNP machine. Cell, 2009, 136(4): 701-18.
3. Y Shi. The Spliceosome: A Protein-directed Metalloribozyme. J Mol Biol., 2017.







